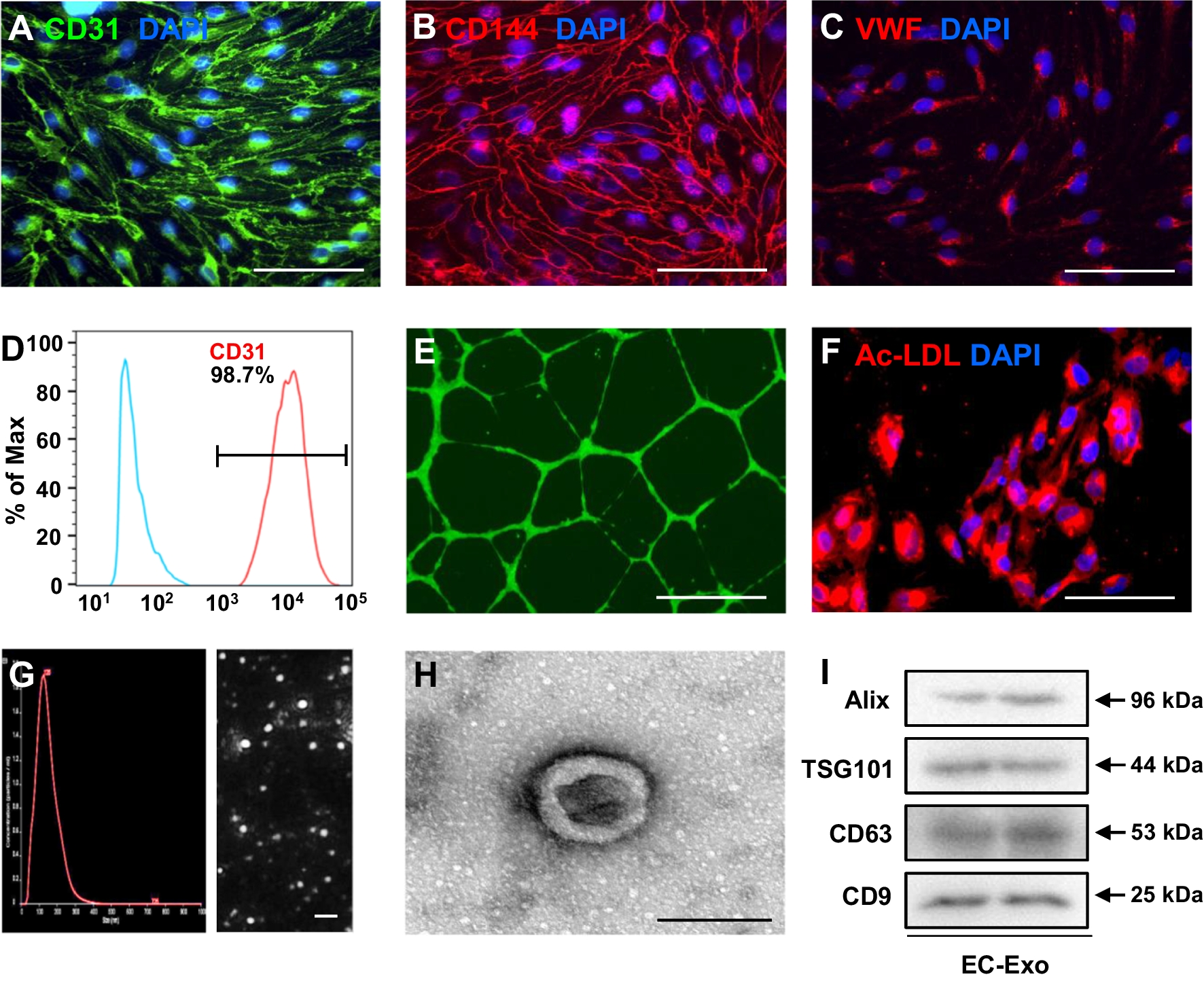
Human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) exhibit the potential to repair the injured heart after myocardial infarction (MI) by promoting neovascularization and cardiomyocyte survival. However, because of the low cellular retention and poor engraftment efficacy, cell therapy of MI is partly mediated by exosomes secreted from the transplanted cells. In this study led by Tongji University, they investigated whether exosomes secreted from hiPSC-ECs could become a promising acellular approach to repair the infarcted heart after MI and elucidated the underlying protective mechanism. The hiPSC-ECs were differentiated, and exosomes were isolated in vitro. Then, hiPSC-EC exosomes were delivered by intramyocardial injection in a murine MI model in vivo. Echocardiography, combined with hemodynamic measurement, histological examination, Ca2+ transient and cell shortening assessment, and Western blot, was used to determine the protective effects of hiPSC-EC exosomes on the infarcted heart. Furthermore, LC Sciences’ microRNA sequencing service, luciferase activity assay, and microRNA gain–loss function experiments were performed to investigate the enriched microRNA and its role in exosome-mediated effects. In vitro, the hiPSC-EC exosomes enhanced intracellular Ca2+ transients, increased ATP content, and improved cell survival to protect cardiomyocytes from oxygen–glucose deprivation injury. Congruously, hiPSC-EC exosome administration in vivo improved the myocardial contractile function and attenuated the harmful left ventricular remodeling after MI without increasing the frequency of arrhythmias. Mechanistically, the hiPSC-EC exosomes notably rescued the protein expression and function of the sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA-2a) and ryanodine receptor 2 (RyR-2) to maintain intracellular Ca2+ homeostasis and increase cardiomyocyte contraction after MI. The microRNA sequencing showed that miR-100-5p was the most abundant microRNA in exosomes. miR-100-5p could target protein phosphatase 1β (PP-1β) to enhance phospholamban (PLB) phosphorylation at Ser16 and subsequent SERCA activity, which contributes to the hiPSC-EC exosome-exerted cytoprotective effects on maintaining intracellular Ca2+ homeostasis and promoting cardiomyocyte survival. The hiPSC-EC exosomes maintain cardiomyocyte Ca2+ homeostasis to improve myocardial recovery after MI, which may provide an acellular therapeutic option for myocardial injury.
Li Hao, Wang Lu, Ma Teng, Liu Zhongmin, Gao Ling. (2023) Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell Research & Therapy 14(1), 278. [article]
Human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) exhibit the potential to repair the injured heart after myocardial infarction (MI) by promoting neovascularization and cardiomyocyte survival. However, because of the low cellular retention and poor engraftment efficacy, cell therapy of MI is partly mediated by exosomes secreted from the transplanted cells. In this study led by Tongji University, they investigated whether exosomes secreted from hiPSC-ECs could become a promising acellular approach to repair the infarcted heart after MI and elucidated the underlying protective mechanism. The hiPSC-ECs were differentiated, and exosomes were isolated in vitro. Then, hiPSC-EC exosomes were delivered by intramyocardial injection in a murine MI model in vivo. Echocardiography, combined with hemodynamic measurement, histological examination, Ca2+ transient and cell shortening assessment, and Western blot, was used to determine the protective effects of hiPSC-EC exosomes on the infarcted heart. Furthermore, LC Sciences’ microRNA sequencing service, luciferase activity assay, and microRNA gain–loss function experiments were performed to investigate the enriched microRNA and its role in exosome-mediated effects. In vitro, the hiPSC-EC exosomes enhanced intracellular Ca2+ transients, increased ATP content, and improved cell survival to protect cardiomyocytes from oxygen–glucose deprivation injury. Congruously, hiPSC-EC exosome administration in vivo improved the myocardial contractile function and attenuated the harmful left ventricular remodeling after MI without increasing the frequency of arrhythmias. Mechanistically, the hiPSC-EC exosomes notably rescued the protein expression and function of the sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA-2a) and ryanodine receptor 2 (RyR-2) to maintain intracellular Ca2+ homeostasis and increase cardiomyocyte contraction after MI. The microRNA sequencing showed that miR-100-5p was the most abundant microRNA in exosomes. miR-100-5p could target protein phosphatase 1β (PP-1β) to enhance phospholamban (PLB) phosphorylation at Ser16 and subsequent SERCA activity, which contributes to the hiPSC-EC exosome-exerted cytoprotective effects on maintaining intracellular Ca2+ homeostasis and promoting cardiomyocyte survival. The hiPSC-EC exosomes maintain cardiomyocyte Ca2+ homeostasis to improve myocardial recovery after MI, which may provide an acellular therapeutic option for myocardial injury.
Li Hao, Wang Lu, Ma Teng, Liu Zhongmin, Gao Ling. (2023) Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell Research & Therapy 14(1), 278. [article]