Chronic inflammation is critical in the onset and progression of atherosclerosis (AS). The lipopolysaccharide (LPS) level in the circulation system is elevated in AS patients and animal models, which is correlated with the severity of AS. Inspired by the underlying mechanism that LPS could drive the polarization of macrophages toward the M1 phenotype, aggravate inflammation, and ultimately contribute to the exacerbation of AS, LPS in the circulation system was supposed to be the therapeutic target for AS treatment. In the present study led by Huazhong University of Science and Technology, polymyxin (PMB) covalently conjugated to PEGylated liposomes (PLPs) were formulated to adsorb LPS through specific interactions between PMB and LPS. LC Sciences’ RNA Sequencing service was used on total RNA obtained from bone marrow- derived macrophages. In vitro, the experiments demonstrated that PLPs could adsorb LPS, reduce the polarization of macrophages to M1 phenotype and inhibit the formation of foam cells. In vivo, the study revealed that PLPs treatment reduced the serum levels of LPS and pro-inflammatory cytokines, decreased the proportion of M1-type macrophages in AS plaque, stabilized AS plaque, and downsized the plaque burdens in arteries, which eventually attenuated the progression of AS. This study highlighted LPS in the circulation system as the therapeutic target for AS and provided an alternative strategy for AS treatment.
The mechanism underlying the inhibitive effect of PLPs on LPS-induced macrophage activation
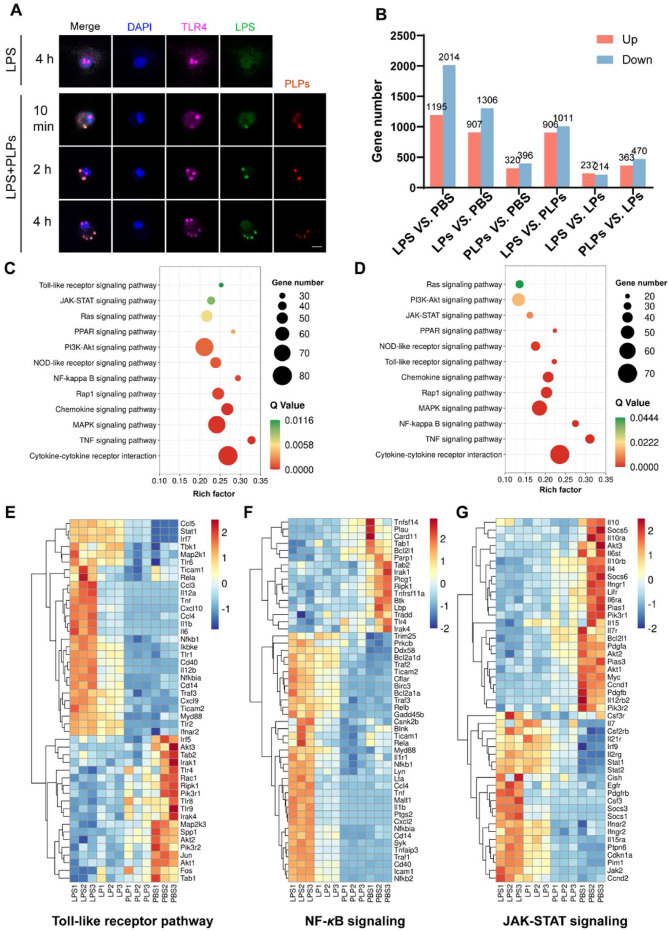
(A) Fluorescence images of macrophages treated with LPS alone or mixed with PLPs over time. The cells were stained with anti-TLR4 antibodies on the surface and counterstained with DAPI for the nucleus. Scale bar = 10 μm. Green: FITC-labeled LPS. Red: DiD-labeled PLPs. Purple: Cy3-labeled anti-TLR4 antibodies. Blue: DAPI-stained nucleus. (B) The number of differentially expressed genes (DEGs) in macrophages between different treatment groups. (C) KEGG enrichment analysis illustrating significantly enriched signaling pathways in LPS versus PBS groups based on DEGs. (D) Based on DEGs, KEGG enrichment analysis illustrates significantly enriched signaling pathways in LPS versus PLPs groups. (E–G) Heatmap of hierarchical cluster analysis displaying normalized gene expression related to Toll-like receptor signaling, NF-κB signaling, and JAK-STAT signaling by different treatments.
Liu Huiwen, Wang Honglan, Li Qiyu, Wang Yiwei, He Ying, Li Xuejing, Sun Chunyan, Ergonul Onder, Can Füsun , Pang Zhiqing, et. al. (2023) LPS adsorption and inflammation alleviation by polymyxin B-modified liposomes for atherosclerosis treatment. Acta Pharmaceutica Sinica B 13(9), 3817-3833. [article]
Chronic inflammation is critical in the onset and progression of atherosclerosis (AS). The lipopolysaccharide (LPS) level in the circulation system is elevated in AS patients and animal models, which is correlated with the severity of AS. Inspired by the underlying mechanism that LPS could drive the polarization of macrophages toward the M1 phenotype, aggravate inflammation, and ultimately contribute to the exacerbation of AS, LPS in the circulation system was supposed to be the therapeutic target for AS treatment. In the present study led by Huazhong University of Science and Technology, polymyxin (PMB) covalently conjugated to PEGylated liposomes (PLPs) were formulated to adsorb LPS through specific interactions between PMB and LPS. LC Sciences’ RNA Sequencing service was used on total RNA obtained from bone marrow- derived macrophages. In vitro, the experiments demonstrated that PLPs could adsorb LPS, reduce the polarization of macrophages to M1 phenotype and inhibit the formation of foam cells. In vivo, the study revealed that PLPs treatment reduced the serum levels of LPS and pro-inflammatory cytokines, decreased the proportion of M1-type macrophages in AS plaque, stabilized AS plaque, and downsized the plaque burdens in arteries, which eventually attenuated the progression of AS. This study highlighted LPS in the circulation system as the therapeutic target for AS and provided an alternative strategy for AS treatment.
The mechanism underlying the inhibitive effect of PLPs on LPS-induced macrophage activation
(A) Fluorescence images of macrophages treated with LPS alone or mixed with PLPs over time. The cells were stained with anti-TLR4 antibodies on the surface and counterstained with DAPI for the nucleus. Scale bar = 10 μm. Green: FITC-labeled LPS. Red: DiD-labeled PLPs. Purple: Cy3-labeled anti-TLR4 antibodies. Blue: DAPI-stained nucleus. (B) The number of differentially expressed genes (DEGs) in macrophages between different treatment groups. (C) KEGG enrichment analysis illustrating significantly enriched signaling pathways in LPS versus PBS groups based on DEGs. (D) Based on DEGs, KEGG enrichment analysis illustrates significantly enriched signaling pathways in LPS versus PLPs groups. (E–G) Heatmap of hierarchical cluster analysis displaying normalized gene expression related to Toll-like receptor signaling, NF-κB signaling, and JAK-STAT signaling by different treatments.
Liu Huiwen, Wang Honglan, Li Qiyu, Wang Yiwei, He Ying, Li Xuejing, Sun Chunyan, Ergonul Onder, Can Füsun , Pang Zhiqing, et. al. (2023) LPS adsorption and inflammation alleviation by polymyxin B-modified liposomes for atherosclerosis treatment. Acta Pharmaceutica Sinica B 13(9), 3817-3833. [article]