Apolipoprotein (APOE) E4 isoform is a major risk factor of Alzheimer’s disease and contributes to metabolic and neuropathological abnormalities during brain aging. To provide insights into whether APOE4 genotype is related to tau-associated neurodegeneration, this group of researchers from University of Florida have generated human P301S mutant tau transgenic mice (PS19) that carry humanized APOE alleles (APOE2, APOE3 or APOE4). In aging mice that succumbed to paralysis, PS19 mice homozygous for APOE3 had the longest lifespan when compared to APOE4 and APOE2 homozygous mice (APOE3 > APOE4 ~ APOE2). Heterozygous mice with one human APOE and one mouse Apoe allele did not show any variations in lifespan. At end-stage, PS19 mice homozygous for APOE3 and APOE4 showed equivalent levels of phosphorylated tau burden, inflammation levels and ventricular volumes. Compared to these cohorts, PS19 mice homozygous for APOE2 showed lower induction of phosphorylation on selective epitopes, though the effect sizes were small and variable. In spite of this, the APOE2 cohort showed shorter lifespan relative to APOE3 homozygous mice. None of the cohorts accumulated appreciable levels of phosphorylated tau compartmentalized in the insoluble cell fraction. They used LC Sciences’ RNA Sequencing service on total RNA extracted from frozen, pulverized mice forebrains. RNAseq analysis showed that the induction of immune gene expression was comparable across all the APOE genotypes in PS19 mice. Notably, the APOE4 homozygous mice showed additional induction of transcripts corresponding to the Alzheimer’s disease-related plaque-induced gene signature. In human Alzheimer’s disease brain tissues, they found no direct correlation between higher burden of phosphorylated tau and APOE4 genotype. As expected, there was a strong correlation between phosphorylated tau burden with amyloid deposition in APOE4-positive Alzheimer’s disease cases. Overall, their results indicate that APOE3 genotype may confer some resilience to tauopathy, while APOE4 and APOE2 may act through multiple pathways to increase the pathogenicity in the context of tauopathy.
Bulk RNAseq analysis of the forebrains of APOE homozygous mice
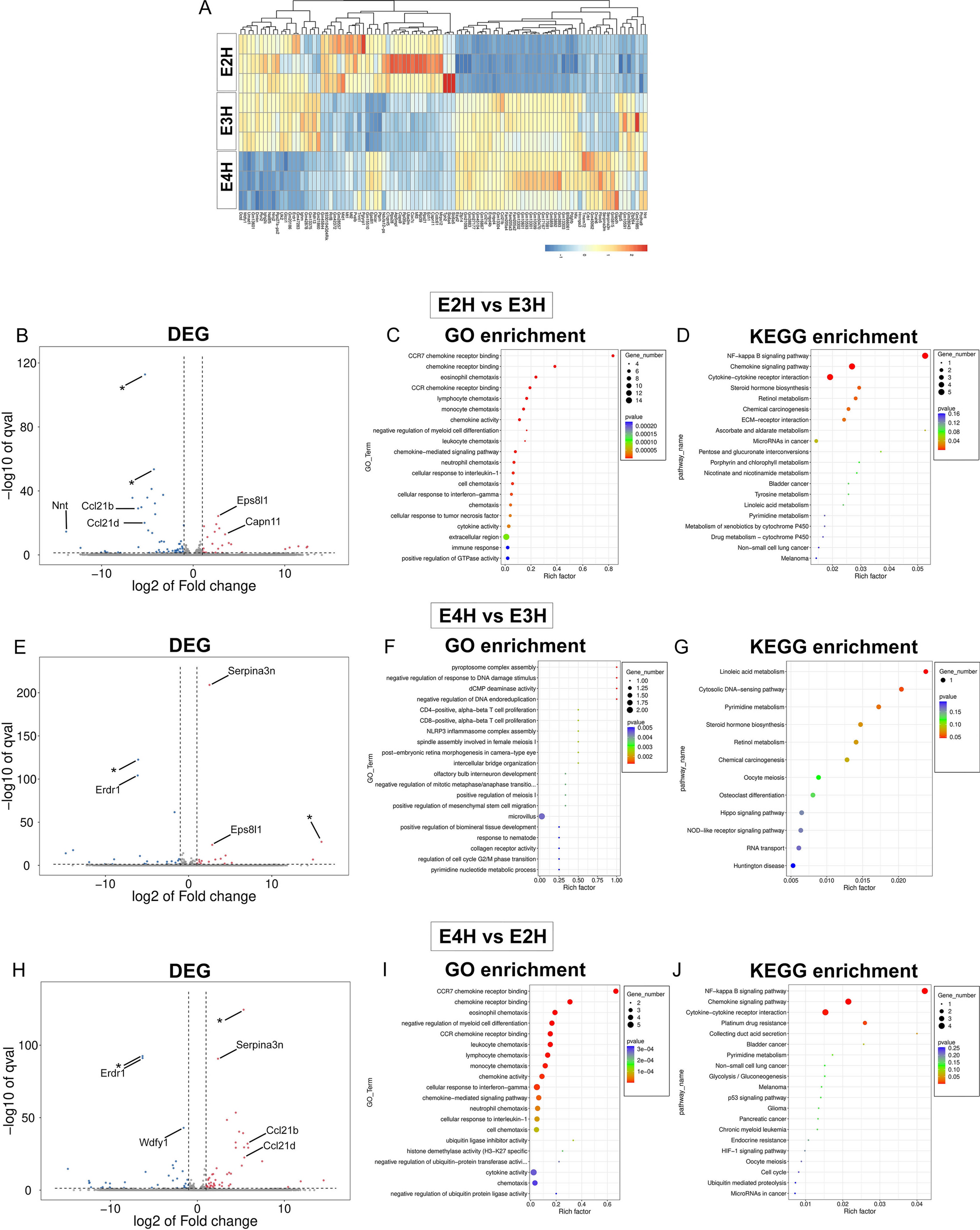
Differentially regulated genes from APOE2, APOE3 and APOE4 homozygous mice are presented in a heat map (A). Volcano plot of DEG and GO and KEGG pathway analysis of enriched gene sets are shown for E2H vs E3H mice (B–D), E4H vs E3H mice (E–G), and E4H vs E2H mice (H–J). Asterisks in volcano plot refer to predicted genes. The bubble plots are colored by p-value and sized by number of genes in enriched gene sets, as defined in the accompanying panel. N = 3 mice/group
Williams Tristan, Bathe Tim, Vo Quan, Sacilotto Patricia, McFarland Karen, Ruiz Alejandra Jolie, Hery Gabriela P., Sullivan Patrick, Borchelt David R., Prokop Stefan. (2023) Humanized APOE genotypes influence lifespan independently of tau aggregation in the P301S mouse model of tauopathy. Acta Neuropathologica Communications 11(1), 99. [article]
Apolipoprotein (APOE) E4 isoform is a major risk factor of Alzheimer’s disease and contributes to metabolic and neuropathological abnormalities during brain aging. To provide insights into whether APOE4 genotype is related to tau-associated neurodegeneration, this group of researchers from University of Florida have generated human P301S mutant tau transgenic mice (PS19) that carry humanized APOE alleles (APOE2, APOE3 or APOE4). In aging mice that succumbed to paralysis, PS19 mice homozygous for APOE3 had the longest lifespan when compared to APOE4 and APOE2 homozygous mice (APOE3 > APOE4 ~ APOE2). Heterozygous mice with one human APOE and one mouse Apoe allele did not show any variations in lifespan. At end-stage, PS19 mice homozygous for APOE3 and APOE4 showed equivalent levels of phosphorylated tau burden, inflammation levels and ventricular volumes. Compared to these cohorts, PS19 mice homozygous for APOE2 showed lower induction of phosphorylation on selective epitopes, though the effect sizes were small and variable. In spite of this, the APOE2 cohort showed shorter lifespan relative to APOE3 homozygous mice. None of the cohorts accumulated appreciable levels of phosphorylated tau compartmentalized in the insoluble cell fraction. They used LC Sciences’ RNA Sequencing service on total RNA extracted from frozen, pulverized mice forebrains. RNAseq analysis showed that the induction of immune gene expression was comparable across all the APOE genotypes in PS19 mice. Notably, the APOE4 homozygous mice showed additional induction of transcripts corresponding to the Alzheimer’s disease-related plaque-induced gene signature. In human Alzheimer’s disease brain tissues, they found no direct correlation between higher burden of phosphorylated tau and APOE4 genotype. As expected, there was a strong correlation between phosphorylated tau burden with amyloid deposition in APOE4-positive Alzheimer’s disease cases. Overall, their results indicate that APOE3 genotype may confer some resilience to tauopathy, while APOE4 and APOE2 may act through multiple pathways to increase the pathogenicity in the context of tauopathy.
Bulk RNAseq analysis of the forebrains of APOE homozygous mice
Differentially regulated genes from APOE2, APOE3 and APOE4 homozygous mice are presented in a heat map (A). Volcano plot of DEG and GO and KEGG pathway analysis of enriched gene sets are shown for E2H vs E3H mice (B–D), E4H vs E3H mice (E–G), and E4H vs E2H mice (H–J). Asterisks in volcano plot refer to predicted genes. The bubble plots are colored by p-value and sized by number of genes in enriched gene sets, as defined in the accompanying panel. N = 3 mice/group
Williams Tristan, Bathe Tim, Vo Quan, Sacilotto Patricia, McFarland Karen, Ruiz Alejandra Jolie, Hery Gabriela P., Sullivan Patrick, Borchelt David R., Prokop Stefan. (2023) Humanized APOE genotypes influence lifespan independently of tau aggregation in the P301S mouse model of tauopathy. Acta Neuropathologica Communications 11(1), 99. [article]