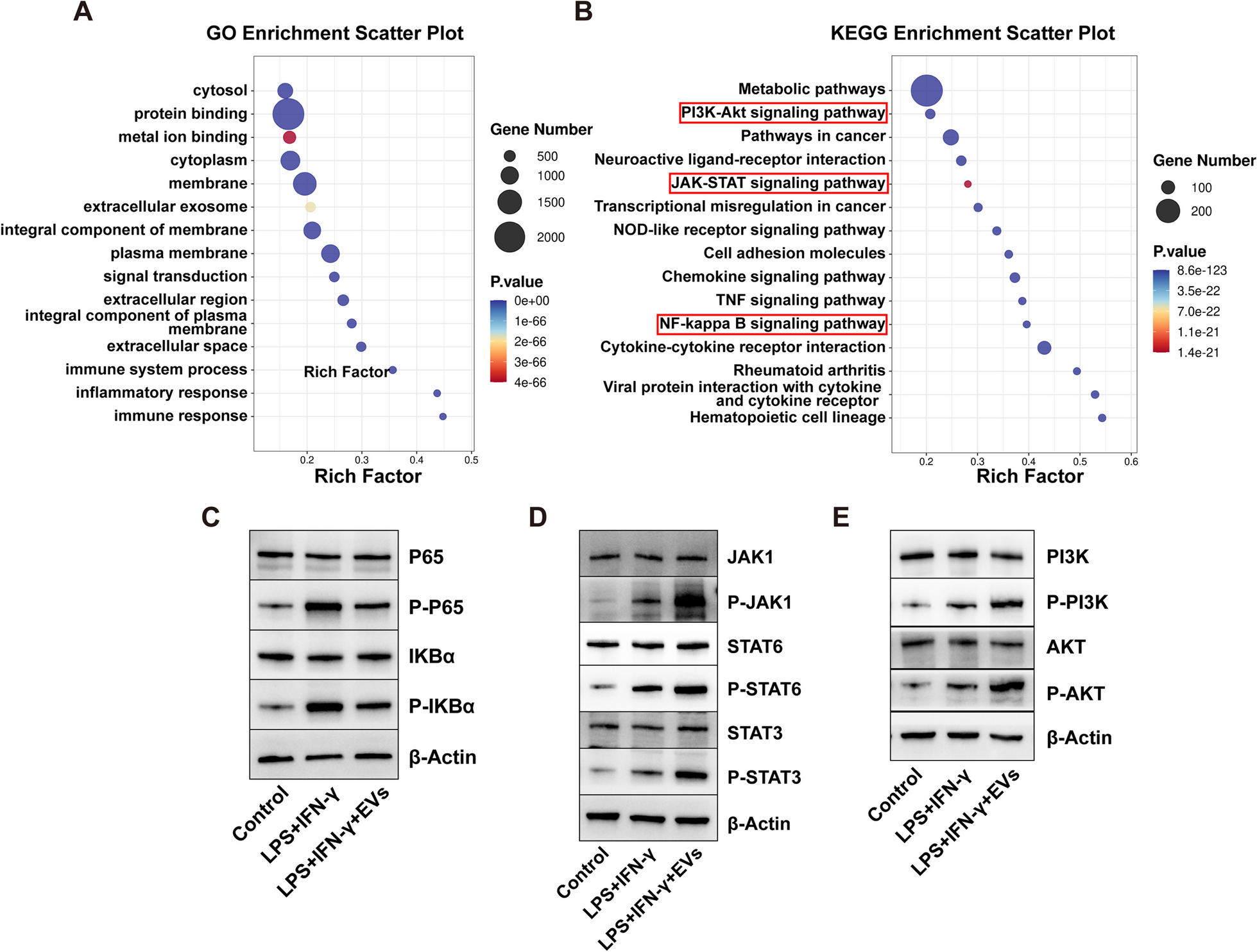
Acute compartment syndrome (ACS) is one of the most common complications of musculoskeletal injury, leading to the necrosis and demise of skeletal muscle cells. The previous study showed that embryonic stem cells-derived mesenchymal stem cells (ESC–MSCs) are novel therapeutics in ACS treatment. As extracellular vesicles (EVs) are rapidly gaining attention as cell-free therapeutics that have advantages over parental stem cells, the therapeutic potential and mechanisms of EVs from ESC–MSCs on ACS need to be explored. In this present study conducted by Zhejiang University, they examined the protective effects in the experimental ACS rat model and investigated the role of macrophages in mediating these effects. Next, they used transcriptome sequencing to explore the mechanisms by which ESC–MSC-EVs regulate macrophage polarization. Furthermore, miRNA sequencing was performed on ESC–MSC-EVs to identify miRNA candidates associated with macrophage polarization. They used LC Sciences’ microRNA sequencing service on total RNA extracted from cell samples. They found that intravenous administration of ESC–MSC-EVs, given at the time of fasciotomy, significantly promotes the anti-inflammation process, angiogenesis, and functional recovery of muscle in ACS. The beneficial effects were associated with ESC–MSC-EVs affecting macrophage polarization by delivering various miRNAs which regulate NF-κB, JAK/STAT, and PI3K/AKT pathways. Their data further illustrate that ESC–MSC-EVs mainly modulate macrophage polarization via the miR-21/PTEN, miR-320a/PTEN, miR-423/NLRP3, miR-100/mTOR, and miR-26a/TLR3 axes. Together, their results demonstrated the beneficial effects of ESC–MSC-EVs in ACS, wherein the miRNAs present in ESC–MSC-EVs regulate the polarization of macrophages.
Jiang Xiangkang, Yang Jingyuan, Lin Yao, Liu Fei, Tao Jiawei, Zhang Wenbin, Xu Jiefeng, Zhang Mao. (2023) Extracellular vesicles derived from human ESC–MSCs target macrophage and promote anti-inflammation process, angiogenesis, and functional recovery in ACS-induced severe skeletal muscle injury. Stem Cell Research & Therapy 14(1), 331. [article]
Acute compartment syndrome (ACS) is one of the most common complications of musculoskeletal injury, leading to the necrosis and demise of skeletal muscle cells. The previous study showed that embryonic stem cells-derived mesenchymal stem cells (ESC–MSCs) are novel therapeutics in ACS treatment. As extracellular vesicles (EVs) are rapidly gaining attention as cell-free therapeutics that have advantages over parental stem cells, the therapeutic potential and mechanisms of EVs from ESC–MSCs on ACS need to be explored. In this present study conducted by Zhejiang University, they examined the protective effects in the experimental ACS rat model and investigated the role of macrophages in mediating these effects. Next, they used transcriptome sequencing to explore the mechanisms by which ESC–MSC-EVs regulate macrophage polarization. Furthermore, miRNA sequencing was performed on ESC–MSC-EVs to identify miRNA candidates associated with macrophage polarization. They used LC Sciences’ microRNA sequencing service on total RNA extracted from cell samples. They found that intravenous administration of ESC–MSC-EVs, given at the time of fasciotomy, significantly promotes the anti-inflammation process, angiogenesis, and functional recovery of muscle in ACS. The beneficial effects were associated with ESC–MSC-EVs affecting macrophage polarization by delivering various miRNAs which regulate NF-κB, JAK/STAT, and PI3K/AKT pathways. Their data further illustrate that ESC–MSC-EVs mainly modulate macrophage polarization via the miR-21/PTEN, miR-320a/PTEN, miR-423/NLRP3, miR-100/mTOR, and miR-26a/TLR3 axes. Together, their results demonstrated the beneficial effects of ESC–MSC-EVs in ACS, wherein the miRNAs present in ESC–MSC-EVs regulate the polarization of macrophages.
Jiang Xiangkang, Yang Jingyuan, Lin Yao, Liu Fei, Tao Jiawei, Zhang Wenbin, Xu Jiefeng, Zhang Mao. (2023) Extracellular vesicles derived from human ESC–MSCs target macrophage and promote anti-inflammation process, angiogenesis, and functional recovery in ACS-induced severe skeletal muscle injury. Stem Cell Research & Therapy 14(1), 331. [article]